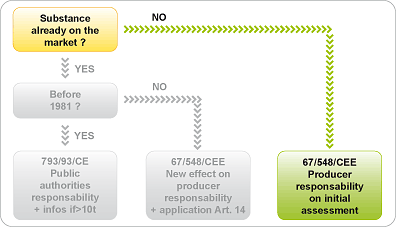
"Nano" form is considered as a new product.
If the nanomaterial is regarded as a substance that has never been marketed, its dangerousness must be assessed and communicated by the person responsible for placing it on the market to the competent authority of the State concerned (Directive 67/548/EEC and Labor Code R.231-52).
Simplified files for progressive thresholds (10 kg, 100 kg, 1 tons/year) and more complete files are planned for higher thresholds (10, 100, 1 000 tons/year and by manufacturer).


