"Nano" form is considered as a classic form.
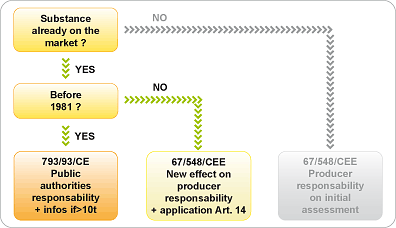
If the nanomaterial is regarded as a substance already on the market, the applicable regulation depends on the date it was placed on the market:
- placed on the market after 1981, the substance has already been the subject of an assessment that probably does not take into account the possible dangers specific to a "nano" form. Article 14 of Directive 67/548/EEC and the Labor Code R.231-52-12 stipulate that the reporter must notify the competent authority of all new information on the effects of the substance on health,
- placed on the market before 1981, the assessment of the dangerousness is the responsibility of the public authorities (793/93/CE). The Commission shall designate each Member State as reporter for the assessment of certain substances, on the basis of information collected from industrialists.
There are only 150 actual evaluations on the 100 000 cases present on the market. Article 7 of Regulation 793/93/CE specifies that a manufacturer who has provided information on a substance, for which more than 10 tons/year have been placed on the market, must notify the competent authorities as soon as he becomes aware of new toxic effects.


