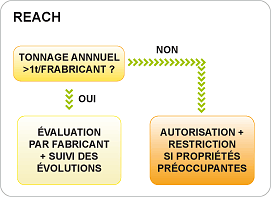
REACH: a tonnage criteria.
For a tonnage > 1 ton/year/establishment, the manufacturer evaluates the dangerousness of the substance and files a report for an examination at the European Chemicals Agency (Helsinki). This report should be updated in case of evolving customs and affects on health.
For a tonnage < 1 ton/year/establishment, a priori, no registration is required. Nevertheless, regardless of the tonnage, a substance with properties of concern (CMR*, PBT*, vPvB*) must be authorized and have a procedure of restriction. The European Agency may limit or prohibit its marketing.
CMR: Carcinogen agent, Mutagen, toxic for reproduction
PBT: Persistent, Bioaccumulable, and Toxic Substance
vPvB : very Persistent and very Bioaccumulable Substance


